Over the last few years, the establishment of innovative therapeutic treatment of solid tumors has markedly ameliorated the outcome of this dreadful pathology, with advances in both the early detection and the survival rate.
Unfortunately, among the various tumor forms, Pancreatic Ductal Adenocarcinoma (PDAC) is not following this trend and still remains one of the deadliest tumor types. In 2021, only in US, an estimated 60,500 adults were diagnosed with PDAC, with an increase in the incidence each year since 2000. The survival rate is tremendously low, 10% at 5 years. For metastasized PDAC, the 5-year survival rate falls to 3%. Moreover, PDAC is, most of the time, very difficult to diagnose; only 11% of PDAC diagnoses are in the early stages. PDAC is relatively resistant to most [] traditional agents, such as gemcitabine, taxanes, and platin-derivatives. For this reason, surgery is considered as the most valuable treatment; however, only 20% of patients are operable1.
This dramatic scenario strongly suggests the need for groundbreaking therapeutic approaches for the treatment of PDAC, and perhaps advanced therapies can be a valuable response to this need. One of the most innovative features of Advanced Therapy Medicinal Products (ATMPs) is to sense the input signals generated by the microenvironments and coherently adapt their response. This potential could possibly bypass the therapeutic barriers of small-molecules and antibodies in the cure of several pathologies.
Considering this concept and considering also that strategies able to modify the tumor microenvironment could considerably ameliorate the pathology outcome, prof. Dominici’s group is actively developing a new gene therapy using engineered Adipose Mesenchymal Stem Cells (AD-MSC) for the cure of PDAC. In the last few years, AD-MSCs has attracted attentions in ATMPs research for their easy accessibility from different sources and for the possibility of their extensive in vitro expansion and gene modification.
In their recent work, prof. Dominici’s team have engineered AD-MSCs to express a soluble form of the potent anti-cancer agent TRAIL. This approach combines the great affinity of MSCs for the tumor microenvironment with the physiological capability of TRAIL to activate the immune system against tumor cells. Scientific observation has highlighted that soluble TRAIL secerned by AD-MSCs were able to induce apoptosis in PDAC cells in vitro, and further were able to control tumor growth without toxicity in vivo2.
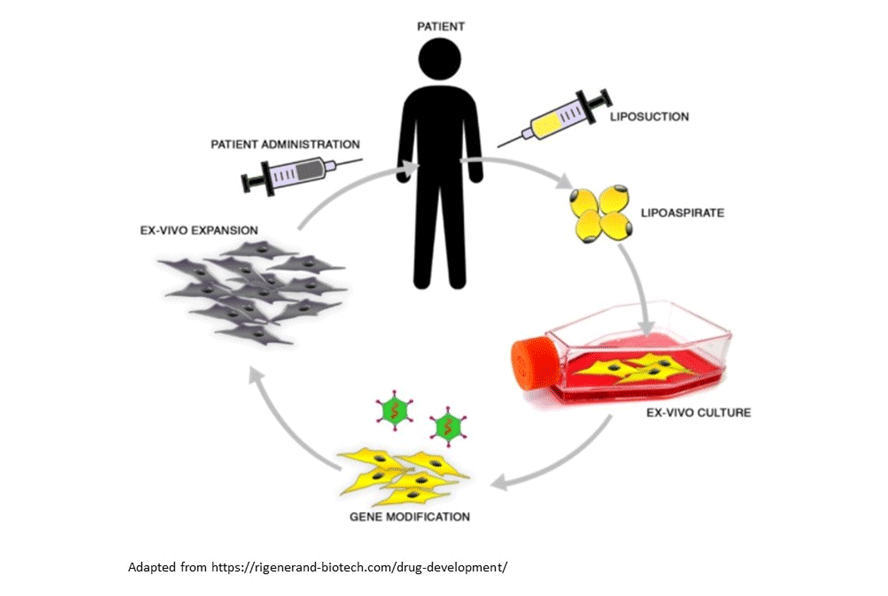
Interestingly, these scientific evidences are now the basis of a new preclinical solution called RR001, developed by a spinoff of University of Modena, Rigenerand. The RR001 technology, formally classified by EMA as gene therapy, is using AD-MSCs isolated through a minimally invasive procedure from the adipose tissue of the patient. These cells are engineered to stably express TRAIL, then expanded and reinfused in the patient to treat the tumor.
This innovative approach is brilliantly utilizing the newest and most advanced technologies to overcome one of the most important barriers of modern medicine, and represents one of the brightest examples of the astonishing potentials of ATMPs in the field of cancer medicine.
Recent Articles
- Cell therapy based on neuronal precursors for the treatment of multiple sclerosis 10 January 2023
- How to Improve the Sustainability of Advanced Therapies: The Case of Strimvelis 24 November 2022
- FDA reorganization: a “Super Office” to manage the increasing cell and gene therapy workload 3 November 2022
- GMP Cleanliness Classifications: Deciphering the Differences and Requirements among Grades 12 September 2022
- How to Overcome the Most Common Issues in the GMP-Compliant Culturing of Mesenchymal Stem Cells: Isolation and Automatization 30 August 2022