Advanced therapy medicinal products, drugs based on biological materials such as tissues, genes, or cells, possess the ground-breaking potential to revolutionize the treatment of several diseases, considered, nowadays, incurables. In the last years, the use of Mesenchymal Stem Cells (MSCs) received great attention for their possible applications in a plethora of different pathologies. MSCs are pluripotent stem cells very well known for their pro-regenerative, pro-vascular, and immunomodulatory properties. Moreover, they can be isolated from accessible sources such as bone marrow and umbilical cord and can differentiate into bone tissue, adipose tissue, or cartilage. However, despite the very high number of investigations and early-stage clinical trials published, only a small fraction of the potential drugs based on MSCs reached the last phases of testing.
Most of the reasons associated with this point can be correlated with the lack of standardized and consistent manufacturing, especially in the final stages of research, when the production rate drastically increases. This lead one hand to high variability in every produced batch, and on the other to extremely high cost for the production, the trained personnel, and the validation.
The isolation and expansion of MSCs is a complex process, requiring stringent controls of every step performed. Traditionally, cells are treated in open system exposed to the external environment and, thus, to contaminations. For this reason, this type of facility required infrastructures able to ensure air exchange, air filtration, barriers, and controlled access.
A possible solution could be found in two concepts that are nowadays considered pivotal in the design of facilities assigned to ATMP production: the isolation of the manufacturing, and the automatization of complex processes.
The isolation of the manufacturing refers to the physical separation between the biological sample and/or the manufacturing procedure and the external environment through the use of a closed system. By definition, a closed system is an internal space physically separated from the extern where there is no contact between the process and the operator. The best application of closed systems is the Isolators, working stations that are used in substitution of the classical Grade B cleanrooms. According to guidelines, isolators can be installed in Grade D rooms, with a series of advantages that can be examined in deep here.
The automatization of complex processes can be considered as an evolution of the concept of automatization: more than merely automatized simple tasks, nowadays scientists and engineers are willing to automatize very complex assignments, such as the expansion of MSCs using bioreactors. Bioreactors are stand-alone cell culture tools equipped with sensors; they are able to monitor and control key parameters such as temperature, pH, and dissolved oxygen (DO). In this way, the complex process of seeding and expanding MSCs previous to the fill and finish procedures can be automatized, standardizing every produced batch. Moreover, bioreactors lead to a significant cost reduction and a great speed-up of the process. In this sense, VivaBioCell bioreactor NANT001 can represent an optimal solution: this bioreactor has been designed to be a closed system, totally isolated from the external environment. Thus, this type of product can be used in a Grade D area.
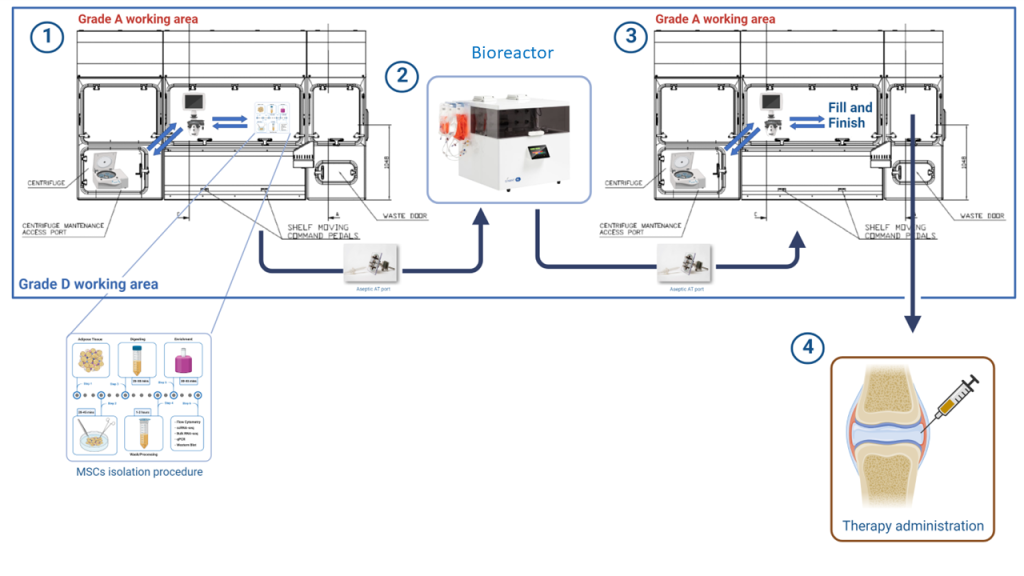
Combining these two concepts we could speculate about an ATMP production process using isolators and bioreactors in a Grade D cleanroom (Fig 1). MSCs could be extracted from tissue in a GMP-compliant way inside an isolator and then moved to the NANT001 bioreactor through aseptic connectors for the expansion phase. After expansion, cells can be moved back to the isolator and the final fill and finish passages can be performed. In this innovative facility, the biological product is always isolated from the external environment and in grade A. Moreover, the automatization of long and complex processes on one hand greatly reduces the costs, on the other speed up production.
Recent Articles
- Cell therapy based on neuronal precursors for the treatment of multiple sclerosis 10 January 2023
- How to Improve the Sustainability of Advanced Therapies: The Case of Strimvelis 24 November 2022
- FDA reorganization: a “Super Office” to manage the increasing cell and gene therapy workload 3 November 2022
- GMP Cleanliness Classifications: Deciphering the Differences and Requirements among Grades 12 September 2022
- How to Overcome the Most Common Issues in the GMP-Compliant Culturing of Mesenchymal Stem Cells: Isolation and Automatization 30 August 2022

