Gene and cell therapies, commonly termed ATMPs represent the cutting-edge of modern medicine, holding the potential to revert the progression of many different pathologies such as Alzheimer’s disease, Parkinson’s disease, cancer, muscular dystrophy, and many others.
One of the most interesting examples of EMA and FDA-approved ATMP is Voretigene neparvovec, commercially named Luxturna. This drug is widely considered as a watershed in the progression of advanced therapies, as it is the first directly administered gene therapy approved in the U.S. that targets a disease caused by mutations in a specific gene. Moreover, Luxturna is an excellent example of the application of concepts and technologies widely used in scientific research and finally translated into the medical field.
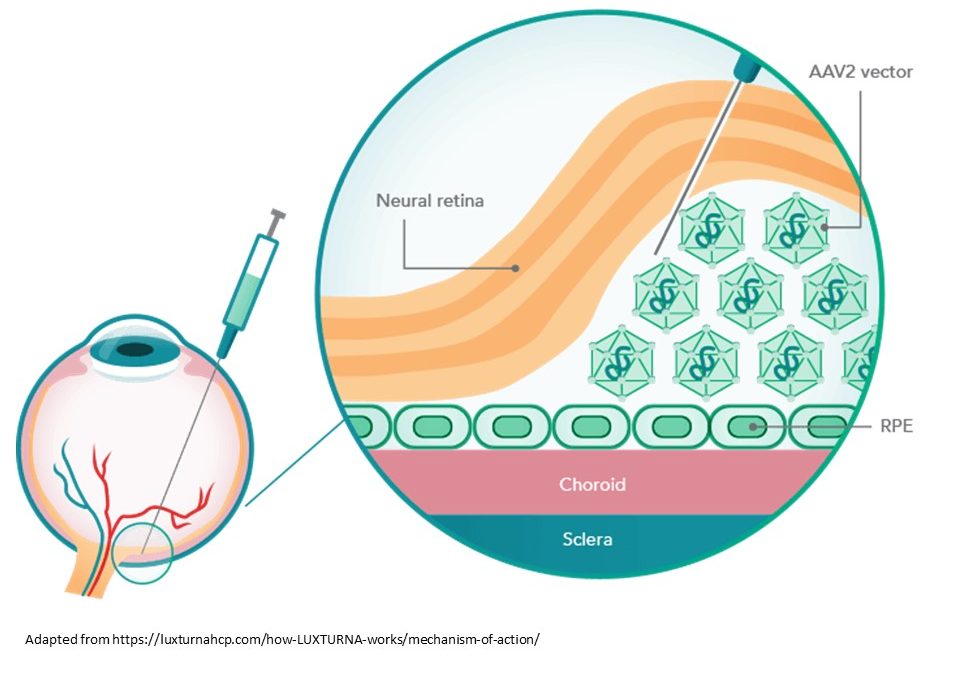
Developed by Spark Therapeutics and Children’s Hospital of Philadelphia, Luxturna is used to treat Leber’s congenital amaurosis, an inherited retinal disease leading to progressive blindness. The cause is the homozygotic mutation of the gene RPE65, expressed in the retinal pigment epithelium and encoding an enzyme fundamental for the visual cycle. Although is not a definitive cure for the condition, this gene therapy treatment substantially improves the vision of affected patients.
Luxturna is formed by an adeno-associated viral vector serotype 2 (AAV2) that carries a functional copy of the RPE65. This copy is linked to a Kozak consensus sequence, a nucleic acid motif that functions as the protein translation initiation site. The drug is administrated through subretinal injection following a vitrectomy. Briefly, the AAV2 carries the copy of the RPE65 gene into the retinal pigment epithelial cells. Herein the vector binds the nucleus and releases the content, producing the RPE 65 enzyme.
As always occurs in all the fast-expanding and newborn fields of medicine, Luxturna gene therapy is far from being a “perfect” solution. In fact, the exorbitant cost (nearly one million dollars for each patient) and the specificity for only one type of genetic disease, reduce the effectiveness of this therapy. Nevertheless, Luxturna has to be truly considered as a turning point in advanced therapy, not only because of highlighting the potentials of ATMPs application, but also because it has allowed researchers to acquire a fundamental experience in the production, test, and manufacture of this type of drug.
Recent Articles
- Cell therapy based on neuronal precursors for the treatment of multiple sclerosis 10 January 2023
- How to Improve the Sustainability of Advanced Therapies: The Case of Strimvelis 24 November 2022
- FDA reorganization: a “Super Office” to manage the increasing cell and gene therapy workload 3 November 2022
- GMP Cleanliness Classifications: Deciphering the Differences and Requirements among Grades 12 September 2022
- How to Overcome the Most Common Issues in the GMP-Compliant Culturing of Mesenchymal Stem Cells: Isolation and Automatization 30 August 2022