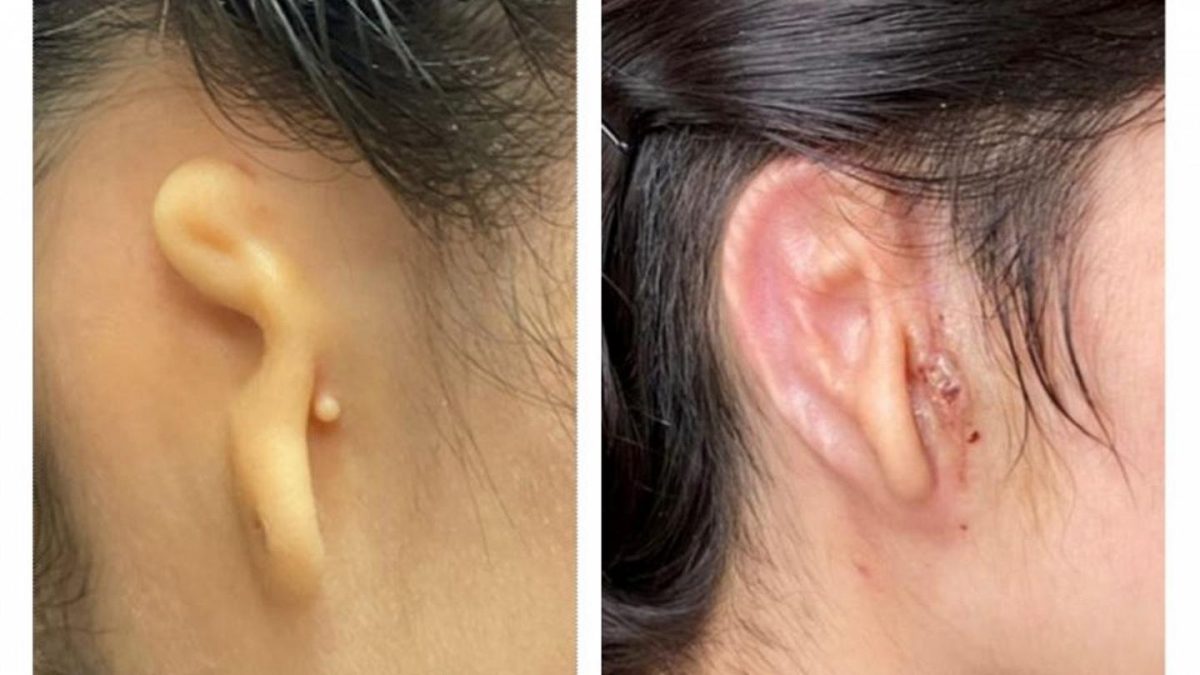
The first 3D bio-printed ear, made of the patient’s own cells, has been recently transplanted in a successful operation. The reconstruction appears to be the first 3D implant-made patient’s living cells to be transplanted into a human patient. Scientifically speaking, the novelty, other than to be the in the transplant per se, is in the structure of the implant: the use of the patient’s own cells collected from the patient’s biopsy and expanded in vitro.
The implant has been developed 3DBio Therapeutic, a clinical-stage biotechnology company based in New York, and present in the market from seven years. The company’s ultimate goal is to deliver safe, functional, and personalized 3D-bioprinted living tissues and organs, engineered on-demand for patients.
The patient who received the transplant, a 20-year-old woman from Mexico, is affected by microtia, a rare congenital disease causing the absence of one of the ears. According to the Centers for Disease Control and Prevention, this pathology affects 1 in every 2,000 to 10,000 born just in the U.S. The causes remained unknown, although some studies correlated this pathology with the use of isotretinoin during pregnancy.
But how did 3DBio Therapeutic perform the first 3D-printed ear using living tissues?
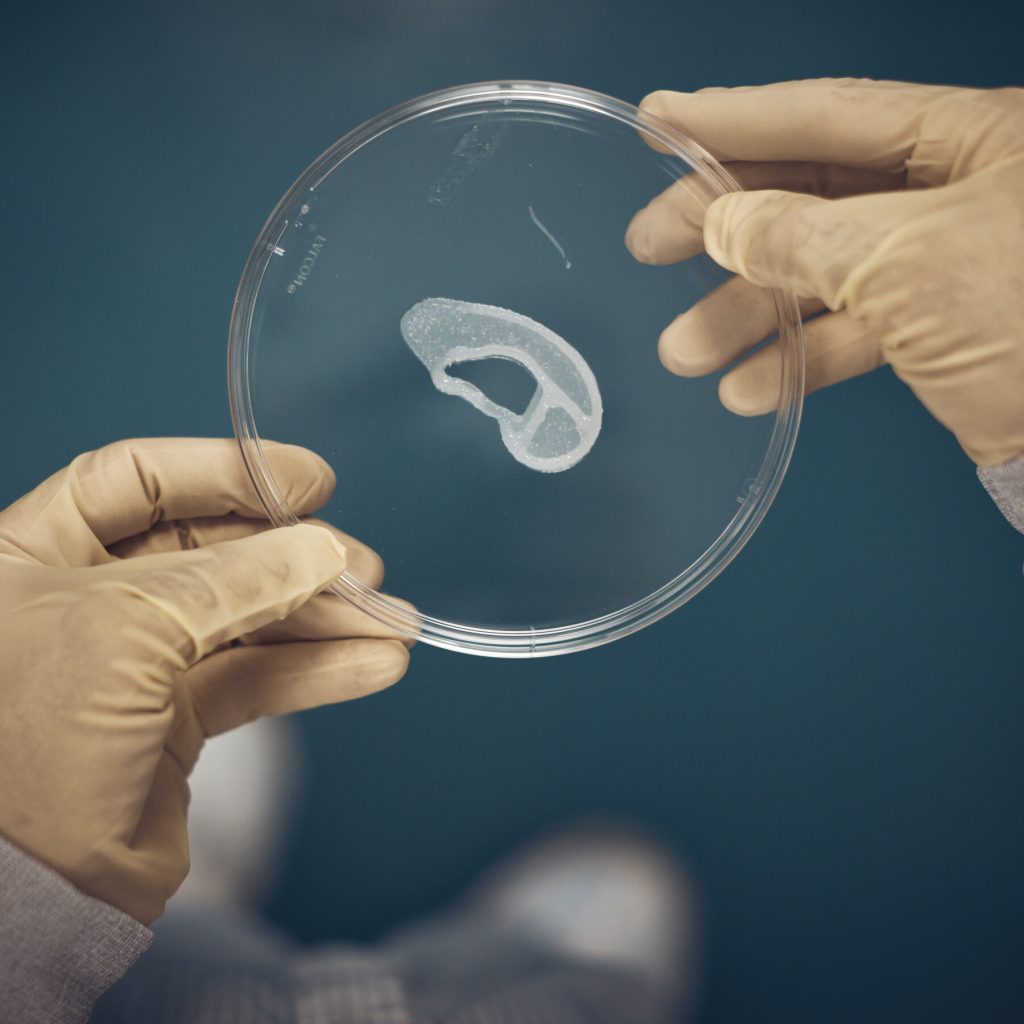
Firstly, the doctors conducted a biopsy on the ear of the patient aiming to isolate chondrocytes, cells specialized in creating cartilage. Those cells were expanded using a specialized cell culture system that ensured rapid cell expansion. The expanded cells were then incorporated into a novel bio-ink, called ColVivo™. The bio-ink has been used as the bases for the printing, performed by the GMPrint™ developed by 3DBio Therapeutic. The scaffold used is the so-called Overshell, a non-permanent structural support to the biological implant. The last step was the surgical transplant, performed by Dr. Arturo Bonilla, a pediatric surgeon at the Congenital Ear Institute, the largest pediatric microtia center in North America.
Traditionally, the transplanted ear is made of rib cartilage, harvested from the patient and requiring at least two separate hours-long procedures. This process, long and invasive, could result in chest deformity and other complications. Another possibility is to use porous polyethylene (PPE) implants, which required taking a large section of skin from a patient’s scalp.
The use of patient’s own cartilage results in a way less invasive procedure, reducing, at the same time, the costs of all the surgical operations and the risk for infection and implant shattering.
This incredible success is just the last of several recent results performed in the field of ATMP used for organ preservation. In January, surgeons transplanted a genetically modified pig’s heart into a human patient affected with critical heart disease.
The novelty procedure utilized by 3DBio Therapeutic combined the cell expansions with the manufacturing in a grade AinB clean room. Interestingly, a well-established way to greatly reduce the costs and diminish all the risks of contaminations can be the use of isolators AinD. This closed system solution allows a physical separation between the operator (that can easily work in a Class D environment) and the bioproducts, sealed in a Class A internal space. Moreover, isolators are flexible and adaptable to the installation of 3D bioprinters and other types of instruments.
Recent Articles
- Cell therapy based on neuronal precursors for the treatment of multiple sclerosis 10 January 2023
- How to Improve the Sustainability of Advanced Therapies: The Case of Strimvelis 24 November 2022
- FDA reorganization: a “Super Office” to manage the increasing cell and gene therapy workload 3 November 2022
- GMP Cleanliness Classifications: Deciphering the Differences and Requirements among Grades 12 September 2022
- How to Overcome the Most Common Issues in the GMP-Compliant Culturing of Mesenchymal Stem Cells: Isolation and Automatization 30 August 2022