Advanced Therapies Medicinal Products (ATMPs) are medicines or drugs based on cells or gene therapies. In order not to alter their peculiar properties, ATMPs cannot be sterilized, and thus cleaning procedures before, during, and after the manufacturing process are fundamental to ensure the right grade of safety for both bioproducts and operators. One of the most used methods to overcome this particular issue is the use of isolators, closed systems that allow physical isolation between the working area (grade A of sterility) and the external environments (grade D). These structures should be preferred to the classical white rooms (open systems) for the ATMPs production, as suggested by Eudralex (Vol. 4, Annex 4).
As a corollary of such awareness, the definition of decontaminations, disinfection, sterilization, and all the connected terms are essential and, for this reason, in the following part, various useful explanations are listed.
Decontamination (or Decon) is the process of cleansing an object or substance to remove contaminants such as micro-organisms or hazardous material, including chemicals, radioactive substances, and infectious diseases.
Disinfection is defined as the destruction of micro-organisms, except bacterial spores, on inanimate objects (if they deal with living tissue, this is antisepsis). Three levels of disinfection are achievable depending on the amount and kind of microbial killing involved. These levels of disinfection are as follows:
- High Disinfection Level (HDL): the destruction of all viruses, vegetative bacteria, fungi, mycobacteria, and some, but not all, bacteria spores.
- Intermediate Disinfection Level (IDL): the destruction of all mycobacteria, vegetative bacteria, fungal spores, and some nonlipid viruses, but not bacterial spores.
- Low Disinfection Level (LDL): a process that can kill most bacteria (except mycobacteria or bacterial spores), most viruses (except some nonlipid viruses), and some fungi.
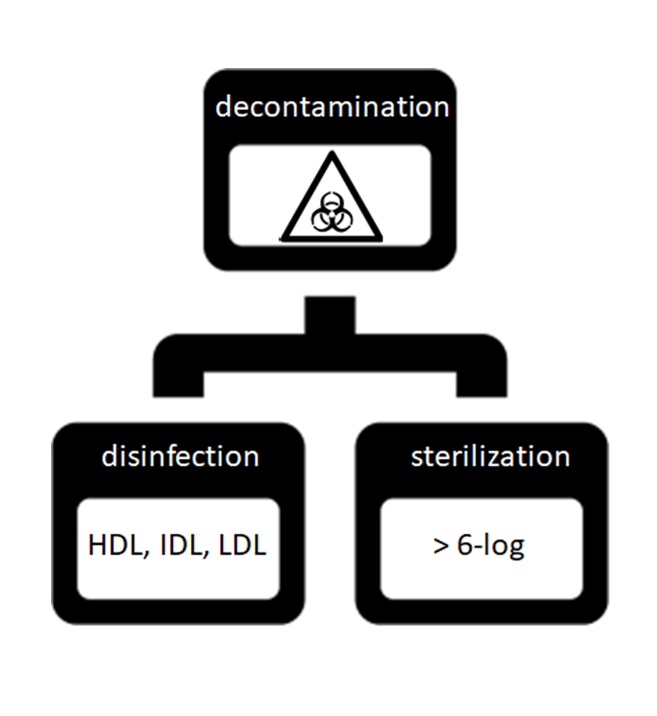
Sterilization refers to any process that removes, kills, or deactivates all forms of life (viruses, bacteria, fungi, spores, etc…) and other biological agents, like prions, present in a specific surface, object, or fluid, for example, food or biological culture media.
Disinfection and Sterilization are a form of Decontamination as shown in the figure.
Cleaning is the process of removing residues and soil (dirt, dust, protein, skin cells, and so on) from surfaces to the extent they are visually clean. Cleaning ensures that a disinfectant will work effectively and interact with the microbial cells. A detergent is a chemical that penetrates soiling and reduces the surface tension (which fixes the soil to the surface) to allow its removal. Cleaning can remove or dilute microbial population and many detergents contain chemical additives that can “disinfect”, but a detergent is not a disinfectant.
Log Reduction is a measure of how thoroughly a decontamination process reduces the concentration of a contaminant. A “log reduction” (technically a reduction by one order of magnitude) represents a 90% reduction in microbial population. As a “rule of thumb” the sterilization target is usually at least a 6-log reduction of micro-organism (spore) population. 6-log reduction mean a 99.9999% reduction of the microbial population.
D-Value (or decimal value) is the time required, at a given condition or set of conditions, to achieve a log reduction, that is, to kill 90% (or 1 log) of relevant micro-organisms. Higher D-Value means higher resistance of the microbial strain to the decontamination process. The D-value is a function of sterilization conditions and varies with the type of micro-organism, temperature, water activity, pH, etc…
Bio-decontamination. A 6-log reduction is a common goal of bio-decontamination, whereas the goal of “sterilization” is to kill all microorganisms, viruses, and spores. Bio-decontamination is used due to its relative safety for operators, equipment, and materials and the bio-decontaminated status can be achieved through different methods:
- Heat: Steam (autoclave), Dry Steam.
- Chemical Sterilization: Ethylene Oxide (EtO), Hydrogen Peroxide (H2O2), Formaldehyde, Chlorine Dioxide (ClO2).
- Radiation: Ionizing Radiation (ɣ-ray), non-ionizing radiation (UV).
- Filtration.
Adapted from the original article by Alessandro Tornani.
Recent Articles
- Cell therapy based on neuronal precursors for the treatment of multiple sclerosis 10 January 2023
- How to Improve the Sustainability of Advanced Therapies: The Case of Strimvelis 24 November 2022
- FDA reorganization: a “Super Office” to manage the increasing cell and gene therapy workload 3 November 2022
- GMP Cleanliness Classifications: Deciphering the Differences and Requirements among Grades 12 September 2022
- How to Overcome the Most Common Issues in the GMP-Compliant Culturing of Mesenchymal Stem Cells: Isolation and Automatization 30 August 2022

